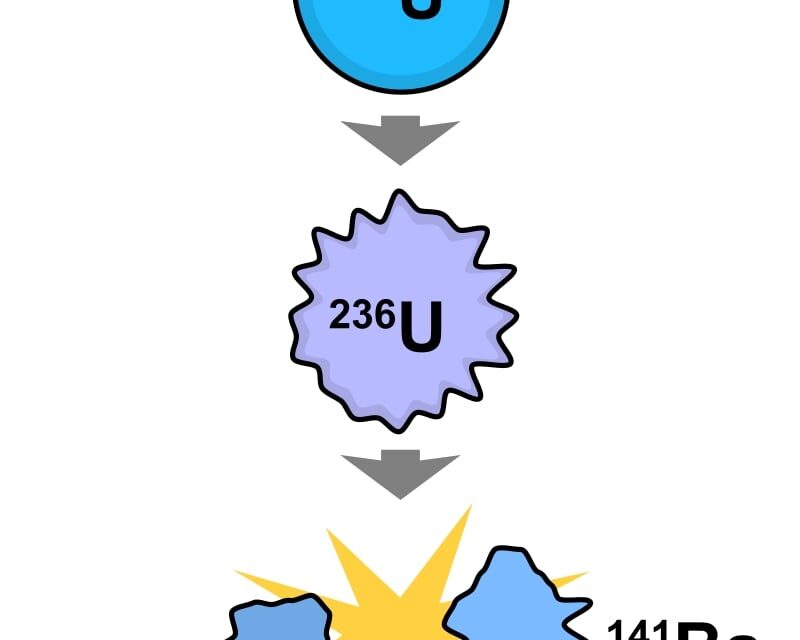
Uranium-235 (235U) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238It is fissile, i.e., it can sustain a nuclear chain reaction. The only fissile isotope that exists in nature as a primordial nuclide.
Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about 584.3±1 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236.
Read this article full with Pritish Kumar and get enough knowledge about Uranium.
Fission properties
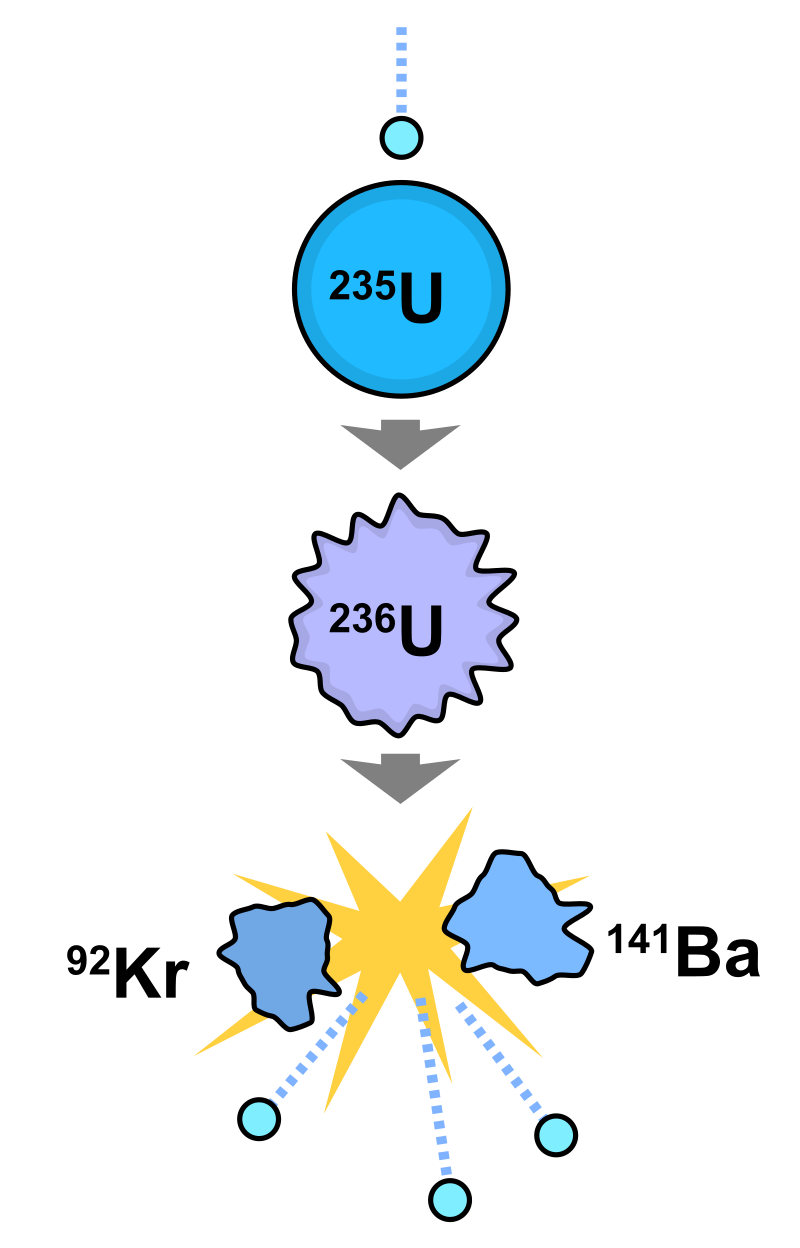
The fission of one atom of uranium-235 releases 202.5 MeV (3.24×10−11 J) inside the reactor. That corresponds to 19.54 TJ/mol, or 83.14 TJ/kg.[3] Another 8.8 MeV escapes the reactor as anti-neutrinos. When uranium 235 nuclides are bombarded with neutrons, one of the many fission reactions that it can undergo is the following
Nuclear fusion
Heavy water reactors and some graphite moderated reactors can use natural uranium, but light water reactors must use low enriched uranium because of the higher neutron absorption of light water. Uranium enrichment removes some of the uranium-238 and increases the proportion of uranium-235. Highly enriched uranium (HEU), which contains an even greater proportion of uranium-235, is sometimes used in the reactors of nuclear submarines, research reactors and nuclear weapons.
If at least one neutron from uranium-235 fission strikes another nucleus and causes it to fission, then the chain reaction will continue. If the reaction continues to sustain itself, it is said to be critical, and the mass of 235U required to produce the critical condition is said to be a critical mass. A critical chain reaction can be achieved at low concentrations of 235U if the neutrons from fission are moderated to lower their speed, since the probability for fission with slow neutrons is greater.
A fission chain reaction produces intermediate mass fragments which are highly radioactive and produce further energy by their radioactive decay. Some of them produce neutrons, called delayed neutrons, which contribute to the fission chain reaction. The power output of nuclear reactors is adjusted by the location of control rods containing elements that strongly absorb neutrons, e.g., boron, cadmium, or hafnium, in the reactor core. In nuclear bombs, the reaction is uncontrolled and the large amount of energy released creates a nuclear explosion.
Uses
Uranium-235 has many uses such as fuel for nuclear power plants and in nuclear weapons such as nuclear bombs. Some artificial satellites, such as the SNAP-10A and the RORSATs were powered by nuclear reactors fueled with uranium-235.
Reference
https://en.wikipedia.org/wiki/Uranium-235